Optical sensing for
early cardiovascular diagnostics
Janis Spigulis*, Girts Venckus and Maris Ozols
University of Latvia, Department of Physics
and IAPS, Raina Blvd. 19, Riga, LV-1586, Latvia
ABSTRACT
A sensor device for noninvasive detection and analysis of the pulsating blood flow waveforms by means of the reflective single-period photoplethysmography (SPPPG) technique has been designed and clinically tested. The sensor is operated jointly with any standard PC, by connecting the sensor head to the AD-card and using a separate hard disc with the signal processing software; all circuits are feeded by the PC power supply. After processing, normalized shape of the mean SPPPG signal and its parameters are calculated and displayed; the measurement/processing time does not exceed 2 minutes. The clinically detected SPPPG signal shapes and corresponding parameters are presented and discussed. The preliminary results confirm good potential of this sensing approach for fast and patient-friendly early cardiovascular diagnostics.
Keywords: Photoplethysmography, optical bio-sensing, cardiovascular diagnostics.
1.
INTRODUCTION
When the tissue is illuminated by visible or near-infrared cw radiation, heartbeat-period changes in the transmitted and scattered optical signal levels can be recorded by means of the photoplethysmographic (PPG) sensors 1. The PPG signals are originated by absorption of optical radiation by the pulsating blood volume, therefore they contain clinically valuable information on the blood pumping and transport conditions in living body.
A number of transmission-type finger and earlobe PPG devices for monitoring of heartbeat rate and tissue blood supply have been designed and routinely used. Advanced designs of the back-scattering or reflection-type PPG sensors 2 are of increased interest today, mainly thanks to the their clinically more convenient one-touch operation mode. However, the reflected PPG signals are weaker and therefore more noisy than the transmitted ones.
Full and clear clinical interpretation of all components of the PPG signals is still problematic. Qualitatively, one can assume that the initial part of the detected heartbeat signal (raising front and systolic peak) mainly reflects the heart condition and activity, while the following part of a pulse is generally determined by elasticity and other features of the vascular system.
PPG signals are not strictly repeating and periodical, there are slight fluctuations of the signal amplitude, baseline and period 3. Consequently, the fluctuations take place relatively to some virtually stable mean single-period PPG (SPPPG) signal. This mean signal can be identified by averaging a number of sequent PPG pulses over a time interval longer than 50 seconds (which is the longest fluctuation period 3). The recently developed technique of the reflected PPG signal accumulation and integrated processing made possible to detect and analyze the mean SPPPG signals with fairly good accuracy and quality 4-6. Following our initial results, the mean SPPPG signal shape appears to be a very individual feature for each monitored person; qualitative differences in signal shapes for healthy individuals compared to those for persons with cardio-vascular disorders were observed. Obviously, the SPPPG signal shape contains certain coded information regarding the cardio-vascular state of the patient, and a detailed shape analysis eventually might provide clinical data for early cardio-vascular diagnostics in future. To check this opportunity in clinical environment for larger number of patients, more specific sensor design and signal processing technique had to be developed. This paper describes the design and signal processing concepts of our newly developed SPPPG sensor as well as some results of clinical trials carried out with this device.
* E-mail: janispi@latnet.lv

![]()
![]()
![]()
![]()
![]() IBM/PC
IBM/PC
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]() LED
LED
M HD AD
![]()
![]()
![]()





![]()
![]()
![]()
![]()
![]()
![]() R +12V
R +12V

![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]() AD
AD
![]() PD PA
PD PA
![]()
Tissue Contact probe Amplifier AD-card Hard disc Monitor
Fig. 1.
Block-diagram of the optical SPPPG sensor device.
2.
THE SENSOR DESIGN
The advanced SPPPG sensor is intended for clinical use in conjunction with any standard PC. Three basic modules are needed for its operation – the sensor head (fingertip probe with amplifier), standard AD-card and a standard hard disk (preferably separate) with the signal processing software and space for storage of the recorded data. Feeding of all electronic circuits is provided by the PC power supply.
Block-diagram of the device is presented on Fig. 1. The finger contact probe - sensor head - comprises a continuously emitting diode LED (GaAs, lmax ~ 940 nm) integrated with a photodiode PD (Si, 1 cm2 active area) and a pre-amplifier chip PA. The pre-amplified PD output signal was passed via a flexible cable to the broadband amplifier and further to the AD-card input. Only the ac component of the photodiode output signal was amplified and further processed; the amplifier provided about 400-fold magnification. Following results of our recent study 4, frequency filtering of the remitted PPG signals may cause their shape-deformations, therefore signals of all frequencies were amplified and passed to input of the 12-byte AD-card.
The LED and photodiode of the fingertip probe were down-oriented during the measurements, and additional calibrated load was applied to provide equal pressure force (0.65 N) to the fingertip skin for all monitored patients. The contact probe was placed within a vertical cylindrical capsule, which served simultaneously as a finger-holder, external light shield and sliding guide for the optical probe. The 3rd (middle) finger of left arm was mainly used for the SPPPG signal recording, and the patients were kept in horizontal position (lying on their back) during the measurements.
3. THE SIGNAL PROCESSING
The signal processing software was stored on a separate hard disk, which served for operation of the sensor device and for storage of the measured and calculated data. The algorithm for integration and averaging of the detected PPG signals 4 was updated following our recent experience, and a service program was added. At the beginning of each trial, a window for entering the monitored patient data (name, age, pathology, etc.) was opened. Then a measurement window with instructions appeared, and after proper placing of the fingertip probe the measurements were started. The data were recorded for 60 – 80 seconds, then the whole PPG signal was stored in the HD memory and processed. A special algorithm calculated the mean normalized SPPPG signal for the monitored person and the corresponding signal shape parameters (maxims, minims, amplitude ratios, integral area, etc.). Heartbeat rate and arrhythmia could be calculated, as well. All these data appeared on the PC monitor within less than 5 seconds, so the time necessary for the whole procedure normally did not exceed two minutes.
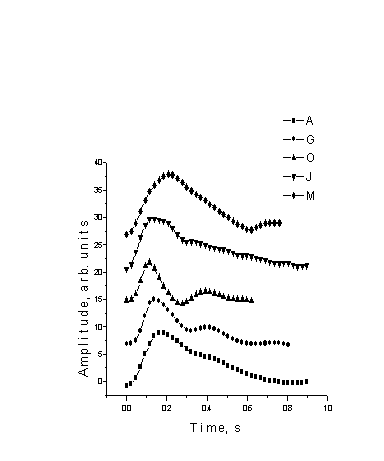
Fig. 2.
Single-period PPG signal shapes for various patients.
4.
RESULTS OF THE CLINICAL
TRIALS
Some of the initially measured mean SPPPG signals for different persons are presented on Fig. 2. The signals were taken at the same location of the body (tip of the left thumb) and accumulated over 80 seconds. The monitored persons were without any pronounced cardio-vascular or any other pathologies. The following abbreviations were used: A, G and O - males of age 24 – 26 years, J - a male of 49, M - a female of 56.
Fig. 2 illustrates clear differences in the SPPPG signal shapes for the five persons. The notch which typically follows the diastolic dip is more pronounced in the cases of younger patients; this might be interpreted as a sign of better vascular elasticity compared to the older patients.
Changes in the SPPPG signal shapes after performing of various physical exercises were observed, too. As expected, the pulse period decreased due to the increasing heartbeat rate, but the initial parts of the SPPPG signals (raising front and time-position of the systolic peak) did not change much.
Clinical trials with the SPPPG sensor device have been started also to patient groups selected accordingly to specific cardio-vascular pathologies; this study is still underway at the Latvian Institute of Cardiology. Several numerical parameters characterizing the mean SPPPG signal shape were calculated and analysed for each monitored person, such as time positions of all maxims and minims, ratios of their amplitudes, etc. For illustration, some of the clinical data are presented on Fig. 3 and Fig. 4.
Fig. 3 illustrates degree of repeatability of the recorded mean SPPPG signals for three healthy individuals. The measurements were repeated after 15-30 minutes, and no physical activities have been undertaken over this time.
Fig. 4 demonstrates the shapes of the mean SPPPG signals recorded in clinical environment for five hypertension patients – four females and one male of age interval 52 …74 years (average of three to five measurement series for each). One can note that the after-systolic notches in the SPPPG signals for all these patients can not be observed at all.
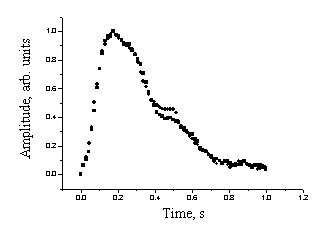
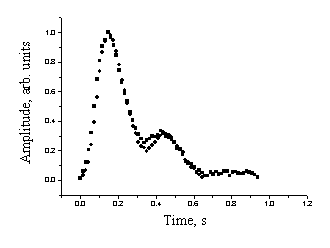
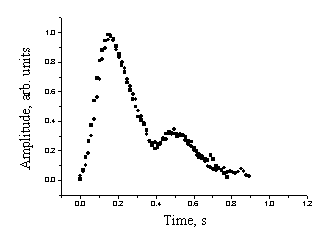
Fig. 3. The
mean SPPPG signal repeatability: two measurement series for three patients.
Fig. 4. The mean SPPPG signal shapes for five
Hypertension patients: clinical records,
lack of the after-systolic notch observed.
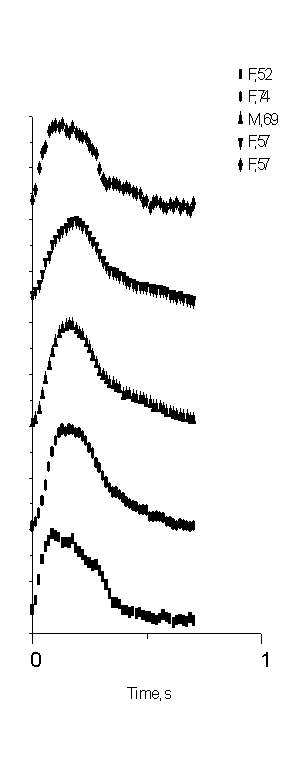
5. CONCLUSIONS
An optical single-period photoplethysmography (SPPPG) sensor device has been designed and clinically tested. Results of the clinical measurements show fairly good quality of the recorded mean SPPPG signals thus opening the possibility of detailed analysis of the signal shapes. The mean SPPPG signal shapes appear to be different and very individual for each monitored person, and it obviously reflects in certain way the cardio-vascular state of this particular person.
Joining the technical (sensor design) efforts with medical expertise and better understanding of the clinical needs, this technique would be successfully used for fast, easy and patient-friendly early diagnostics of cardio-vascular pathologies in the nearest future.
ACKNOWLEDGMENTS
Authors are very grateful to medical doctors Ieva Markowitza and Indulis Kukulis (Latvian Institute of Cardiology) for valuable discussions and arrangements for the clinical trials with the sensor device. Assistance of M. Phys. Uldis Rubins in development and tests of the advanced signal processing software is highly acknowledged.
REFERENCES
1. A. B. Hertzman, “Photoelectric plethysmograph of the finger and toes in man”, Proc. Soc. Exp. Biol. Med. 37, pp. 1633-1637, 1937.
2. H. Ugnell, “Phototplethysmographic Heart and Respiratory Rate Monitoring”, Ph. D. Thesis No. 386, Linkoping University, 1995.
3. M. Nitzan, H. de Boer, S. Turivnenko et al., “Power spectrum analysis of spontaneous fluctuations in the photoplethysmographic signal”, J. Bas. Clin. Physiol. Pharmacol., 5, No. 3-4, 1994, pp. 269-276.
4. J. Spigulis, U. Rubins, “Photoplethysmographic sensor with smoothed output signals”, Proc. SPIE. 3570, 1998, pp. 195-199.
5. G. Venckus, J. Spigulis, “Frequency filtering effects on the single-period photoplethysmography signals”, Med. Biol. Eng. Comput., 37, Suppl. 1, 1999, pp. 218-219.
6. J. Spigulis, G. Venckus, “Single-period photoplethysmography: a potential tool for noninvasive cardiovascular diagnostics”, Springer Series “Optics for Life Sciences” OFLS-VI, Berlin (in press).