Optical multi-channel monitoring of skin blood
pulsations
for cardiovascular assessment
Janis Spigulis*, Renars
Erts and Maris Ozols
ABSTRACT
Time
resolved detection and analysis of the skin back-scattered optical signals
(reflection photoplethysmography or PPG) provide rich
information on skin blood volume pulsations and can serve for cardiovascular
assessment. The multi-channel PPG concept has been developed and clinically
verified in this work. Simultaneous data flow from several body locations
allows to study the heartbeat pulse wave propagation in real time and to
evaluate the vascular resistance. Portable two- and four-channel PPG monitoring
devices and special software have been designed for real-time data acquisition
and processing. The multi-channel devices were successfully applied for
cardiovascular fitness tests and for early detection of arterial occlusions.
Keywords: Photoplethysmography, optical bio-sensing, cardio-vascular
assessment. fitness control.
1.
INTRODUCTION
Photoplethysmography (PPG) is
a non-invasive method for studies of the blood volume pulsations by detection
and temporal analysis of the tissue back-scattered or transmitted optical radiation.
Blood pumping and transport dynamics can be monitored at different body
locations - fingertip, earlobe, forehead, forearm, etc. – with relatively
simple and convenient PPG contact probes. The AC-component of back-scattered
PPG signals reliably reflects skin blood volume pulsations, therefore PPG
technique has good potential to become a routine tool for express diagnostics
and early screening of cardio-vascular pathologies, for self-monitoring at home
and in public facilities, as well as and for the tele-diagnostics
via Internet or LAN.
In general, each recorded PPG
pulse contains useful information for cardio-vascular assessment. More detailed
information can be obtained by analysis of the PPG signal sequences recorded
over some period of time, e.g. one to few minutes. The PPG signal amplitude,
baseline and period are changing with time in result of respiration, neural
activities and body movements 1. One can assume that the real
bio-signals are fluctuating around some stable mean single-period (SP-PPG)
signal that can be determined by averaging a sequence of PPG pulses using
specific algorithms and PC-processing programs. Our first robust SP-PPG
fingertip sensor devices 2, 3 had undergone several series of
clinical tests in laboratory, classroom and hospital environments. Analysis of
fingertip signals taken from a number of volunteers had lead to conclusion that
each person has his/her specific shape of the mean SP-PPG signal,
and this "PPG-fingerprint" reflects the individual's cardio-vascular
condition. Later a portable single-channel PPG sensor model based on a laptop
computer was developed, and new data on cardio-vascular conditions and skin
micro-circulation have been reported 4,
5.
Recently
portable versions of two-channel and four-channel PPG sensor devices have been
developed and tested. These sensors comprise a set of universal optical contact
probes, electronic converters and a laptop computer with specially designed
software to provide real-time display, processing and storage of the PPG signals
that are recorded simultaneously at each channel. The proposed multi-channel PPG approach offers
two basic advantages:
–
increased data flow - more comparative cardio-vascular data can be
recorded and analyzed in real time,
–
additional physiological parameter - vascular resistance
- can be estimated and compared by measurements of time shifts between the
corresponding PPG pulses detected at different anatomical sites.
*) E-mail: janispi@latnet.lv,
tel/fax: +371 7228249
2. DUAL-CHANNEL PPG SENSOR DEVICE
The dual-channel device comprises
two optical contact probes (applied simultaneously during the measurements),
the bio-signal acquisition/conversion circuit and a laptop computer with
specially developed software. All equipment is placed in a hand-held case of
size 44x32x9 cm and weight 4.1 kg; it is battery-powered and can operate up to
3 hours without recharging.


a b
Fig. 1. The dual-channel PPG sensor device (a) and application of the
optical contact probes (b).
Each optoelectronic contact probe
emits cw-radiation into the skin tissues and detects
the back-scattered radiation; the separated AC-component of the signals
precisely reflects the skin blood pulsations at the probe-covered volume. Both
contact probes comprise a pair of GaAs emitting diode
(diameter of the emitting area ~2 mm, radiant power ~10 mW,
peak wavelength ~940 nm, the estimated mean penetration depth under the skin
surface ~2-3 mm) and Si photodiode (square detection
area ~5x5 mm). Both diodes are closely mounted on a soft plastic pillow and
fixed onto the measurement site by means of a sticky band or a finger-clip.
Advantage of the band-probe design is the possibility of its flexible extension
by means of spare sticky bands, so allowing to take the PPG measurements from
practically any location of the body, e.g. forehead, forearm, neck, belly,
calf, etc.
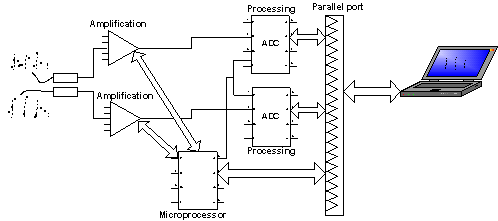
Fig. 2. The dual-channel PPG sensor device: functional scheme.
Special software was developed for the PPG bio-signal
acquisition, processing and data storage from both input channels, offering the
following options:
·
Filling the first window for patient data - name,
age, gender, complains, doctor’s comments, etc.;
·
Pre-setting the measurement time schedule;
·
Real-time display of both PPG signals;
·
Signal clean-up (special filtering algorithm) and
calculation of the mean single-period PPG (SP-PPG) signal shape;
·
Calculation of specific cardio-vascular parameters
for the registered signals - heartbeat rate, anacrota rise-time, time delay
and relative amplitude of the secondary peak (dycrotic notch), time-shift
between two corresponding PPG signals at both channels, etc.;
·
Display of the selected PPG parameter set with
corresponding cardio-vascular assessment results;
·
Storage of the obtained data.
The data sampling rate 100 s -1 was
usually chosen; it could be increased up to 950 s -1 for special
cases when higher time resolution was needed. Screen-shot of the two-channel
PPG measurement process is presented at Fig. 3.Time-shift between the signals of both channels (detected at
the neck artery and fingertip) is clearly observable.
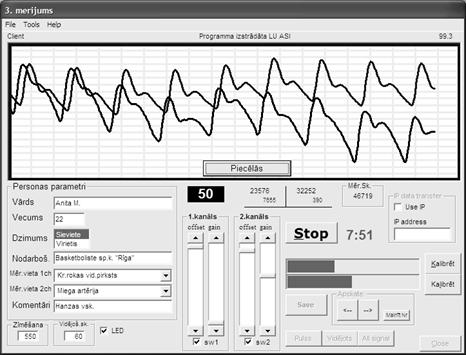
Fig. 3. The monitor screen-shot taken during the
two-channel PPG measurements.
3.
APPLICATION
EXAMPLE: FITNESS TESTS WITH DUAL-CHANNEL PPG SENSING
We performed series of dual-channel PPG measurements
before, during and after intensive physical exercises. The goal was to look for
specific exercise-induced features of bio-signals that might give evidence of
possible cardio-vascular disorders of the monitored person, as well as to
assess his/her adaptability to physical loads. The monitored volunteers – about
200 in total - were persons of different ages, genders and training background,
including about 30 professional sportsmen. The PPG signals were detected
simultaneously from the left middle fingertip and from the Carotid artery area
on the left side of neck.
The test protocol included four stages – 1minute
horizontal relax, 1minute steady standing position, 3 minutes
metronome-controlled stepping up and down (so-called Harvard step-test 6),
and 5 minutes relax in sitting position. The dual-channel PPG signals were
recorded continuously over the whole test, 10 minutes for each volunteer 7,
8.
As result, a Microsoft Access database with Visual Basic
6.0 processing program was created for further analysis of the functional
parameters at different test phases. The pulse rate variations (Fig. 4) were
calculated from the varying time intervals between the neighboring PPG peaks,
the dominant pulse rate oscillation frequencies - using Fourier analysis, the
mean recreation time after the exercise - by finding the time constant of
exponential pulse rate decay, the pulse wave propagation velocity – from the
time shift between two corresponding PPG pulses detected at both channels.
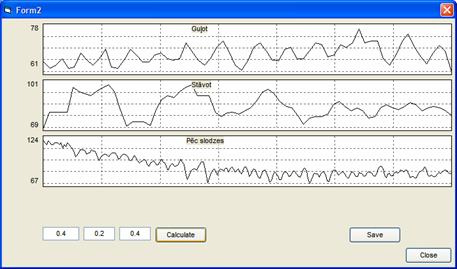
2.
Fig. 4. Data extraction
example: pulse rate variations during the rest phases (horizontal, vertical and
sitting relax).
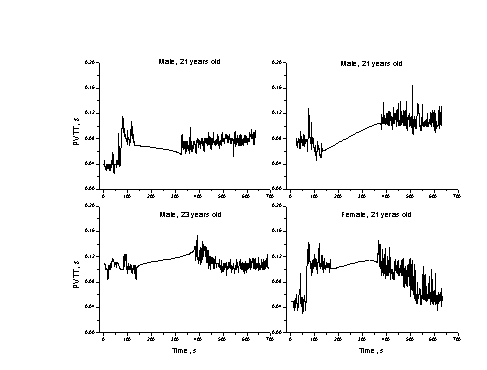
Fig. 5. The variations of pulse
wave transit time during the fitness test (4 volunteers).
Additional advantage of the two-channel approach is
the possibility to verify suspicious features of signals appearing at one
channel by comparing with those detected at the other channel. For instance,
the after-exercise heart arrhythmias (if appeared) had been always convincingly
detected simultaneously at both channels. Following all phases of the test, we
often observed notable variations not only in the pulse rate, but also in the
time-shift between the PPG pulses in both channels, so indicating to changes of
pulse wave transit time (proportional to the vascular resistance) during the
test. Such individual dependencies (Fig. 5) eventually might serve as vascular
health markers, as well.
4.
THE FOUR-CHANNEL PPG
MONITORING: FIRST CLINICAL DATA
Following
similar principles as described for the dual-channel sensor, a more advanced
four-channel PPG device has been designed and clinically tested. Fig. 6
illustrates its basic concept – to provide simultaneous PPG data flow from four
body locations, e.g. the same fingertips of both arms and the same toes of both
legs. Such measurement scheme looks promising for fast detection and/or
monitoring of vascular occlusions that are often located in the arm or leg
arteries. If there is a notable vascular narrowing or occlusion, the vascular
resistance increases and pulse wave propagates slower. Consequently, some time
shift between corresponding PPG signals in the “right finger – left finger” or
the “right toe – left toe” channels should appear; differing PPG signal shapes
are also expected.
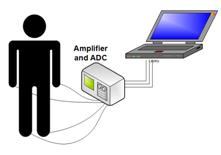
Fig. 6. The four-channel PPG monitoring scheme.
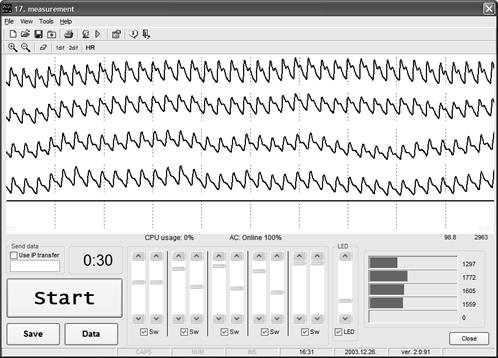
Fig. 7. The
monitor screen-shot taken during the four-channel PPG measurements.
The
4-channel PPG sensing results are represented in real time (Fig. 7); all data
are also stored digitally for their further analysis. Direct on-line display of
the time shift between the PPG peaks of any two selected channels is also
available.
The
four-channel system had undergone its first clinical tests at the Cardiology
Department. As example - a patient, male of age 65, had signs of arterial
occlusion in his left hand (diagnosed by MD after the blood pressure
examinations: 135 + 5 mm Hg for the right hand, 105 + 5 mm Hg for
the left hadnd); his right leg artery had been
occluded and was surgically treated several weeks ago. A fragment of his
4-channel peripheral PPG signals set is presented at Fig. 8, A. There is clear
difference in shapes of both finger-signals (c, d), and also (less notable) of
both toe-signals (a, b). Besides, the signal from the left-hand finger (c) is
delayed relatively to the signal from the right-hand finger (d). Obviously, the
vascular resistance in the left hand is higher - this confirms the occlusion
diagnosis. To compare, in the case of healthy volunteer (Fig. 8, B) the PPG
signal shapes in all four channels were similar and no time shifts between the
finger-finger (c-d) or toe-toe (a-b) signals have been detected.
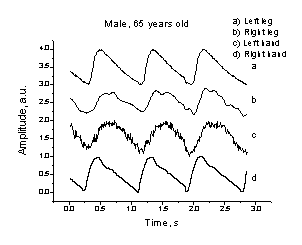
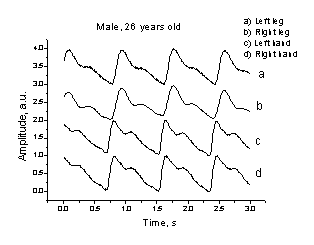
A B
Fig. 8. Comparison of the 4-channel PPG recordings taken from a patient
with signs of the left arm occlusion (A) and from a healthy volunteer (B).
The four contact probes were mounted accordingly to the scheme on Fig. 6.
The
time shift between the right-left fingertip PPG signals in the case of patient
was quite unstable – its changes over half-a-minute are illustrated on Fig. 9,
a. This might be additional feature to confirm increased vascular resistance
causing arterial blood flow irregularities. In the case of healthy volunteer (Fig.
9, b), no time shift within the measurement errors has been detected (note the
error bar).
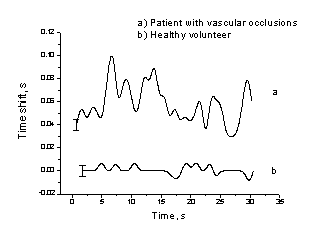
Fig. 7. Variations of the time shift
between PPG pulses detected from the fingertips of both arms:
a – 65-year patient with diagnosis
of one-side arterial occlusion, b – 26-year healthy volunteer.
5. SUMMARY
· The multi-channel PPG concept has been developed by design and clinical tests of portable two- and four-channel sensor devices
· The proposed two-channel PPG methodology and sensor device (44x32x9 cm, 4.1 kg, battery-powered) are well adapted for cardio-vascular monitoring at steady state and during complex fitness tests
· The second PPG channel is important and useful for reference and for obtaining additional physiological data, in particular - time-shift between the PPG pulses simultaneously detected at two different body sites directly reflects vascular blood flow resistance and its changes.
· The newly developed four-channel PPG methodology and sensor device confirmed good potential for early complex diagnosis of arterial occlusions in arms and legs, using as criteria the inter-channel time shifts and signal shape variations
· The discussed technique appears to be well suited for early mass screening and non-invasive monitoring of cardio-vascular disorders at specific conditions where stationary equipment is unavailable.
ACKNOWLEDGMENTS
The authors are sincerely thankful to Dr. Indulis Kukulis for his clinical support and to Prof. Juris Aivars for his valuable physiological comments. Financial support from Latvian Council of Science (grant # 01.0067) and Latvian Ministry of Education and Science (grant # TOP 02-13) is highly appreciated.
REFERENCES
1. M. Nitzan, H. de Boer, S. Turivnenko et al., “Power spectrum analysis of spontaneous fluctuations in the photoplethysmographic signal”, J. Bas. Clin. Physiol. Pharmacol., 5, No. 3-4, pp. 269-276, 1994.
2. J. Spigulis, G. Venckus, M. Ozols, “Optical sensing for early cardiovascular diagnostics”, Proc. SPIE 3911, pp. 27-31, 2000.
3. J. Spigulis, I. Kukulis, E. Fridenberga, G. Venckus, “Potential of advanced photoplethysmography sensing for non-invasive vascular diagnostics and early screening”, Proc. SPIE 4625, pp. 38-43, 2002.
4.
J. Spigulis,
M. Ozols, R. Erts, K. Prieditis, “A portable device for optical assessment of the
cardiovascular condition”, Proc. SPIE 5123, pp. 313-319, 2003.
5.
J.
Spigulis, R. Erts, U. Rubins, “Micro-circulation of skin blood: optical
monitoring by advanced photoplethysmography
techniques”, Proc. SPIE 5119, pp. 219-225, 2003.
6. L. Brouha, A. Graybiel, C. W. Heath, “The step test. A simple method of measuring physical fitness for hard muscular work in adult men”, Rev. Canadian Biol., 2, pp. 86-92, 1943.
7.
J. Spigulis,
R. Erts, V. Bernhards,
“Optics for fitness assessment: potential of two-channel photoplethysmography
techniques”, Abstr. Int. Conf. “Northern
Optics 2003”,
8.
J. Spigulis, R. Erts,
M. Ozols, “A portable two-channel PPG cardiovascular
sensor device”, Proc.
SPIE 5138,
pp. 65-71, 2003.