Potential of advanced photoplethysmography sensing
for non-invasive vascular diagnostics and early
screening
Janis Spigulis*, Indulis Kukulis1,
Eva Fridenberga and Girts Venckus
University of Latvia, Department of
Physics, Raina Blvd. 19, Riga, LV-1586, Latvia
1)
Latvian Institute of Cardiology, Pilsonu 13, Riga, LV-1002, Latvia
ABSTRACT
Advanced sensor device for
shape analysis of the tissue-reflected mean single period photoplethysmography
(SPPPG) signals has been designed and clinically tested. The SPPPG signal shape
reveals individual features of the patient’s cardio-vascular state. Clinical
studies of several patient groups (e.g. diabetes mellitus, atherosclerosis
obliterans, Raynaud’s syndrome) made possible to specify components of the
SPPPG signal that are sensitive to the corresponding organic or functional
pathologies. Comparison of the right and left arm finger SPPPG signal shapes,
for instance, appears to be efficient tool for early screening of unilateral
atherosclerosis obliterans.
Keywords:
Photoplethysmography, optical bio-sensing, diabetes, atherosclerosis, Raynaud's
syndrome.
1. INTRODUCTION
Photoplethysmography
(PPG) is a non-invasive method for studies of the blood volume pulsations by
detection and temporal analysis of the tissue-scattered (-absorbed) optical
radiation. Blood pumping and transport at different body locations - fingertip,
earlobe, forehead, forearm, etc. - are monitored with simple and convenient PPG
contact sensors.
The PPG sensing
technology has been substantially improved since its origins in 19371.
Progress in microelectronics and computer technologies has opened many new
possibilities. For instance, frequency spectra analysis of the PPG signals can
provide valuable information on heart function, respiration, vascular condition
and nervous system 2-8. PPG
is becoming a powerful, safe and easy-to-use tool for express-diagnostics and
early screening of various cardio-vascular pathologies; it can appear useful
for regular self-monitoring of the vascular condition at home or during
individual physical exercises, as well. Tele-diagnostics by means of
PC-connections via Internet or LAN is another area where advanced
PPG-technology becomes very important.
Clear
interpretation of all components at the PPG Fourier spectra is not yet
available. Besides, many doctors prefer visual information (image or diagnostic
curve) rather than complicated frequency spectra. Therefore attempts to obtain
image-based reliable clinical information from the measured PPG signals were
initiated. Basically, each recorded PPG pulse can be used for this purpose;
however, the heartbeat pulses are not equal – the signal amplitude, baseline
and period are significantly changing with time 3. To overcome this,
the mean single-period photoplethysmography (SPPPG) approach was proposed and
investigated at University of Latvia over the few recent years 9-13.
Its main concept is to detect and to accumulate a sequence of 50…80 PPG pulses
with subsequent determination of precise shape of the averaged one-heartbeat
period signal. Special algorithms and PC-processing programs were developed to
obtain the mean SPPPG signals, suitable for further clinical analysis.
Our prototype
SPPPG sensor devices had undergone several series of clinical tests in
laboratory, classroom and hospital environments. Analysis of signals taken from
more than 50 volunteers had lead to conclusion that each individual has his/her
specific shape of the mean SPPPG signal; this "SPPPG-fingerprint"
obviously reflects the individual's cardio-vascular condition. Some recent
clinical results that illustrate potential of this SPPPG methodology for vascular
diagnostics and early screening will be presented and discussed in this paper.
*)
E-mail janispi@latnet.lv
2.
TECHNICAL DETAILS
The basic
details of the SPPPG sensor design and bio-signal processing techniques were
described previously 12. The sensor device consists of the finger
contact probe, signal interface and standard PC. The contact probe comprises
infrared emitting diode, silicon photodiode - detector of the tissue
back-scattered signals, and pre-amplifier. The interface includes signal
amplifier, frequency filter and amplitude-to-digital converter; PC sound card
can be used for the AD-conversion, as well 13. Specially developed
PC software provides the bio-signal storage, processing and display.
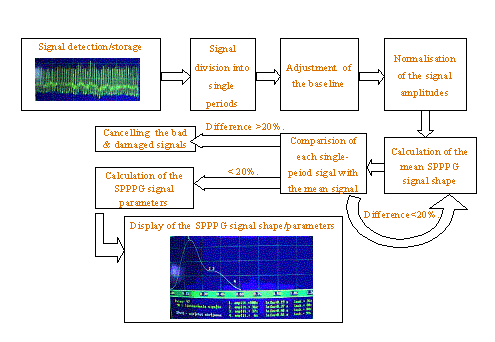
Fig. 1. The mean
SPPPG signal processing scheme.
The algorithm for SPPPG signal processing is illustrated at Fig. 1. After filling-in the patient data, the AC-component of his/her PPG signals is detected and stored; then the whole signal is divided into separate single-period signals, the baseline level is equalized, the single-period signal amplitudes are normalized, and the first approximation of the mean SPPPG signal is calculated. To get rid of occasional fluctuations due to movements, the shape of each measured single-period signal is compared to that of the mean signal. If the amplitude difference at any fixed moment exceeds the threshold value (20 % in most cases), the corresponding measured signals are expelled, but the remaining ones are processed again to calculate the final mean SPPPG signal shape. The specific parameters of the mean SPPPG signal - e.g. time positions of the peaks and dips, interval between primary and secondary peaks, peak amplitude ratio, the dip amplitude related to that of the secondary peak, etc. - are calculated and displayed on the PC monitor, together with the obtained mean SPPPG signal shape. To improve the accuracy, three to five measurement cycles are repeated, and the resulting curve represents average of all taken data. All the recorded PPG signal sequences and the corresponding mean SPPPG signals are stored in the PC memory for further analysis.
3.
RESULTS OF THE CLINICAL TEST MEASUREMENTS
A single-period
PPG signal comprises a fast raising part or anacrota, normally reaching
its peak value within 0.1…0.3 seconds, and a subsequent falling part or catacrota.
Anacrota reflects the stretching of blood vessel walls under the
increased blood pressure after each
heartbeat, and catacrota – relaxation processes of the blood vessel
walls in-between
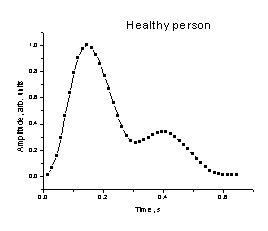
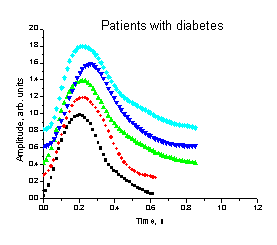
Fig. 2. The mean
SPPPG signal shape for a healthy person. Fig.
3 The mean SPPPG signals taken from fingertips of
five diabetic patients
each two
heartbeats. The catacrota can be variously shaped, depending on the
vascular condition; it normally contains so-called predycrotic dip (which can
be more or less pronounced), and a secondary or dycrotic peak (notch), caused
by elastic reflections in the arterial system. A typical healthy person's mean
SPPPG signal shape is presented at fig. 2.
The shape of single PPG pulse
detected at the periphery (e.g. fingertip) can differ significantly from that
at the magistral arteries; it primarily depends on resistance of the vascular
system. If the vessel resistance is abnormally high due to atherosclerosis,
diabetes or other vascular pathology that narrows the vessels, velocity of
blood flow from big arteries to small capillaries decreases dramatically. As
the result, the propagating blood pressure pulse wave gets broadened and
delayed, and may lose completely its secondary (dycrotic) peak when the
periphery is reached. Absence of the secondary peaks in SPPPG signals taken
from fingertips of the Hypertension patients was a clinical evidence of such
effect 12. Our further studies with five diabetic patients fully
confirmed this assumption - all the SPPPG signals taken from their fingertips
were bell-shaped, without any secondary peak at the catacrota part (Fig.
3).
The clinical trial
with atherosclerotic patients resulted in very similar SPPPG signal shapes.
Effects caused by pharmacological dilatation of the blood vessels by means of
Nitroglycerine were also investigated in this trial. The observed changes
of the mean SPPPG signal shape
with time is presented on Fig. 4, a. One can follow the gradual
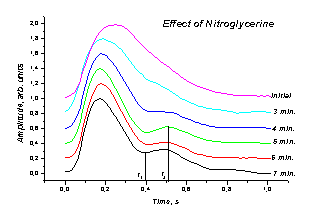
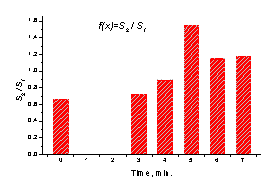
a b
Fig. 4. a - The
mean SPPPG signal changes of asymptotic mild atherosclerotic patient after
taking a Nitroglycrine
dose, b - time development of the
Nitroglycerine effect, characterized by the signal ratio s(t2)/s(t1).
creation and growth of the
secondary peak at the catacrota part of the signal over the first
minutes after in-take of the medicine. It is a clear evidence of increased
blood flow via the damaged vessels due to their enlargement, and confirms possibility of quantitative documentation of this
process by means of the SPPPG techniques. In terms of signal ratio at two fixed
time moments t1 and t2, the blood flow reached its
maximum about 5 minutes after the take-in of Nitroglycerine (Fig. 4, b).
Potential of the
developed SPPPG approach for early screening of the organic pathology in
magistral arteries of arms (obliteration localized in the a subclavia
segment) was confirmed in another clinical study. The previous X-ray tests
and
blood pressure
measurements of the patient had lead to diagnosis that the panga has
been formed at the a subclavia segments of both his arms, but it was
more developed in the right arm. The mean SPPPG signals for this patient were
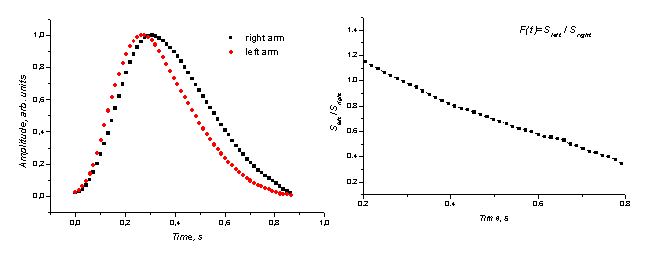
taken from fingertips of both his arms; the results are
presented on Fig. 5, a. A clear time delay and broadening of the right arm signal compared to that of the left arm
is observed, as evidence of
increased vascular resistance and slower
a b
Fig. 5. a -
comparison of the mean SPPPG signals from fingertips of both arms in the case
of obliteration in the a. subclavia (the right arm artery more
occluded), b - the left/right arm SPPPG signal ratio function.
blood flow in
the right arm. The slope angle of the signal ratio function Sleft /
Sright eventually might serve as diagnostic criteria for evaluation
of the blood vessel occlusion (Fig. 5, b).
The Raynod's
syndrome (RS) is a clinical condition characterized by episodic attacks of
vasospasm caused by closure of the most distal parts of the extremities in
response to cold or emotional stress. The female fingers and hands are most
frequently affected. Optical monitoring by PPG and laser-Doppler flowmetry
techniques can provide additional information on this disease 14, 15.
The previous studies 16 have shown that mechanisms involved in the
local regulation of vascular tone (mechano-transduction, energetic metabolism,
endothelium derived factors etc.) could be modified by brief, repetitive
ischemic stress and post-ischemic recovery - preconditioning of the vascular
smooth muscle cells. Seven days long ''training session'' with repetitive (15
times per day) arrest of circulation in the left palm by means of arterial
occlusion for intervals 1to 5 minutes was carried out; the other hand served as
a control.
The SPPPG
monitoring was used to follow vascular changes during such ''training'' of a RS
patient. The mean SPPPG signal shapes of the ''trained'' and control hand were
compared before and after the ''training'' course. The obtained results are
presented at Fig. 6. The notable changes in the SPPPG signal shape may serve as
evidence of improved blood supply in the ''trained'' arm.
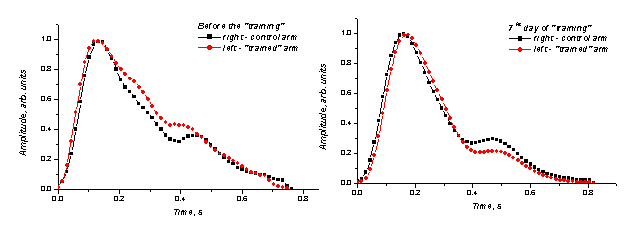
Fig. 6. Comparison of the mean SPPPG signals taken from
fingertips of both hands of the Raynod’s syndrome patient
before and after the periodic
arrest of circulation (''training'') in the left palm.
4.
SUMMARY
The presented
clinical results confirm promising potential of the developed SPPPG sensor
device and methodology for vascular diagnostics and early screening. Several
features of the measured SPPPG signals taken at the fingertip might serve as
the diagnostic/screening criteria:
- raise time of
the anacrota part (time position of the main peak): characterizes the
blood flow resistance in the vessels,
- general shape
of the SPPPG signals: bell-shaped signals without any signs of the dycrotic dip
and peak (notch) at the catacrota part gives evidence of abnormally
narrowed peripheral blood vessels (e.g. diabetes, atherosclerosis),
- appearance and
growth/decrease of the secondary peak as a drug (e.g. Nitroglycerine) response:
monitors the time development of enlargement/narrowing of the blood vessels,
- differences in
shapes of the SPPPG signals taken from both arms: evidence of unilateral
angiosclerotic or other vessel-narrowing pathology,
- changes of the
SPPPG signal shapes in result of physical or physiological (e.g. blood flow
arrest) trainings: reflect the progress of physiological condition.
Hypertension,
diabetes mellitus, atherosclerosis obliterans and Raynod's syndrome patients
have been tested with the SPPPG methodology until now. This non-invasive method
proved to be convenient, fast and reliable, and the developed SPPPG sensor
device – suitable for use in domestic, clinical and laboratory environments.
ACKNOWLEDGEMENTS
The authors
would express their gratitude to engineers Maris Ozols and Renars Erts for
their valuable technical assistance. The financial support from the Latvian
Science Council (Grant # 01.0067) is highly appreciated.
REFERENCES
1. A. B. Hertzman, “Photoelectric plethysmograph of the finger and toes in man”, Proc. Soc. Exp. Biol. Med. 37, pp. 1633-1637, 1937.
2. H. Ugnell, “Phototplethysmographic Heart and Respiratory Rate Monitoring”, Ph. D. Thesis No. 386, Linkoping University, 1995.
3. M. Nitzan, H. de Boer, S. Turivnenko et al., “Power spectrum analysis of spontaneous fluctuations in the photoplethysmographic signal”, J. Bas. Clin. Physiol. Pharmacol., 5, No. 3-4, pp. 269-276, 1994.
4. L. Bernardi, A. Radelli, P. L. Solda et al. “Autonomic control of skin microvessels: assessment by power spectrum of photoplethysmographic waves, Clin. Sci., 90, pp. 345-355, 1996.
5. K. Nakajima, T. Tamura, H. Miike, “Monitoring of heart and respiratory rates by photoplethysmography using a digital filtering technique”, Med. Eng. Phys., 18, 365-372, 1996.
6. P. D. Larsen, M. Harty, M. Thiruchelvam et al, Spectral analysis of AC and DC components of the pulse photoplethysmography at rest and during indication of anaesthesia, Int. J. Clin. Monit. Comput., 14, pp. 89-95, 1997.
7. M. Nitzan, A. Babchenko, B. Khanokh et al., “The variability of the photoplethysmographic signal – a potential method for the evaluation of the autonomic nervous system”, Physiol. Meas., 19, pp. 93-102, 1998.
8. F. Perez-Ocon, A. Abarca, J. Abril et al., “Optical measurement of cardiac rhythm using a personal computer with telediagnosis possibilities”, J. Biomed. Opt., 6, No. 1, pp. 90-96, 2001.
9. J. Spigulis, U. Rubins, “Photoplethysmographic sensor with smoothed output signals”, Proc. SPIE. 3570, pp. 195-199, 1998.
10. G. Venckus, J. Spigulis, “Frequency filtering effects on the single-period photoplethysmography signals”, Med. Biol. Eng. Comput., 37, Suppl. 1, pp. 218-219, 1999.
11. J. Spigulis, G. Venckus, “Single-period photoplethysmography: a potential tool for noninvasive cardiovascular diagnostics”, Springer Series “Optics for Life Sciences” OFLS-VI, Berlin (in press).
12. J. Spigulis, G. Venckus, M. Ozols, “Optical sensing for early cardiovascular diagnostics”, Proc. SPIE 3911, pp. 27-31, 2000.
13. M. Ozols, J. Spigulis, “Acquisition of biosignals using the PC sound card”, Proc. Int. Conf. “Biomedical Engineering” (KTU, Kaunas, LT), pp. 24-27, 2001.
14. A. A. Wouda, “Raynaud’s phenomenon. Photoelectric plethysmography of the fingers of persons with and without Raynaud’s phenomenon during cooling and warming up”, Acta Med. Scand., 201, pp. 519-523, 1977.
15. M. Engelhart, H. V. Nielsen, J. K. Kristensen, “The blood supply to fingers during Raynaud’s attack: a comparison of laser-Doppler flowmetry with other techniques”, Clin. Physiol., 5, pp. 447-453, 1985.
16. I. Kukulis, V. Dzerve, G. Jegorovs et al., ''Effect of repetitive palm blood flow arrest in patients with Raynaud’s phenomenon'', Int. Angiology, 19, Suppl. 1 to Nr. 2, p. 33, 2000.